






《药物合成中的金属有机化学PPT课件下载》是由用户上传到老师板报网,本为文库资料,大小为3.26 MB,总共有60页,格式为pptx。授权方式为VIP用户下载,成为老师板报网VIP用户马上下载此课件。文件完整,下载后可编辑修改。
- 文库资料
- 60页
- 3.26 MB
- VIP模板
- pptx
- 数字产品不支持退货
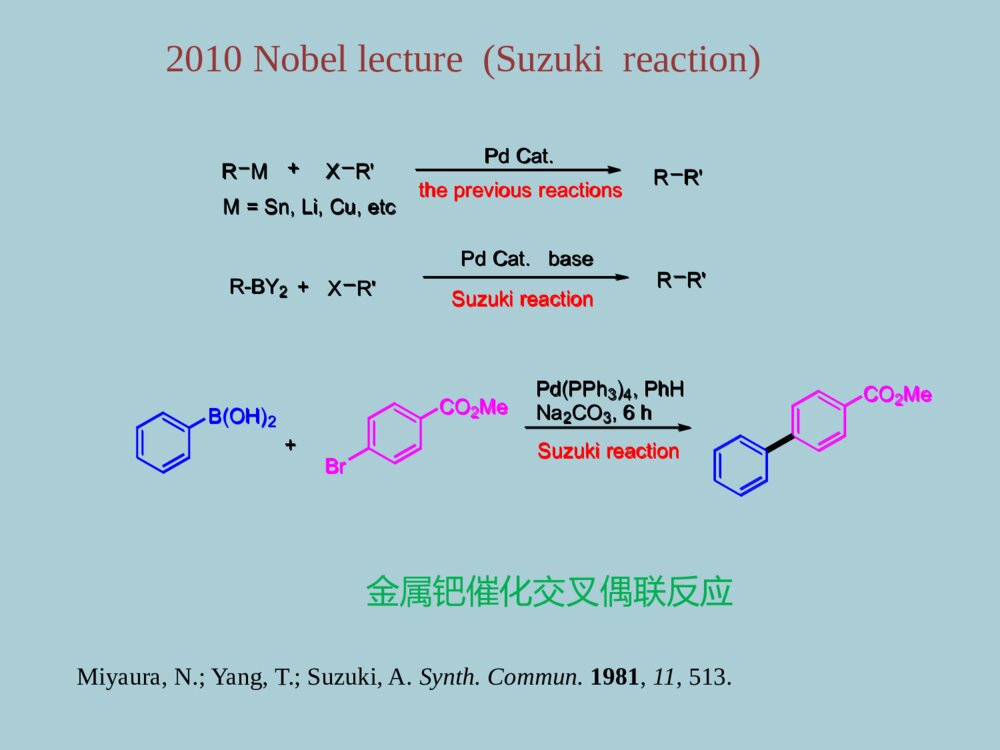
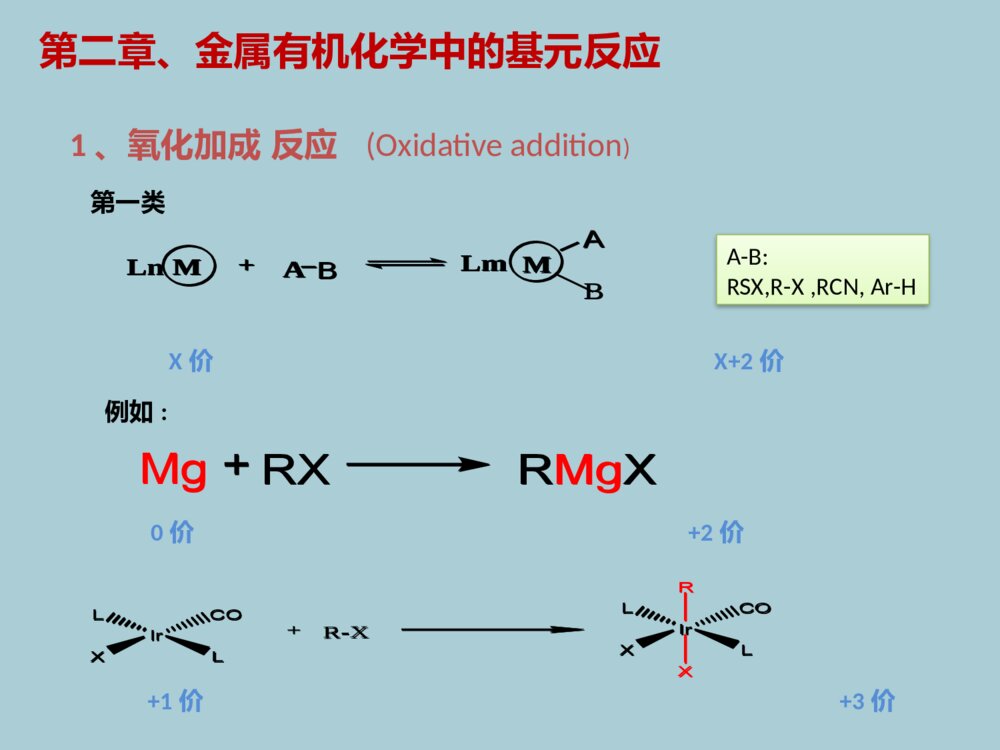
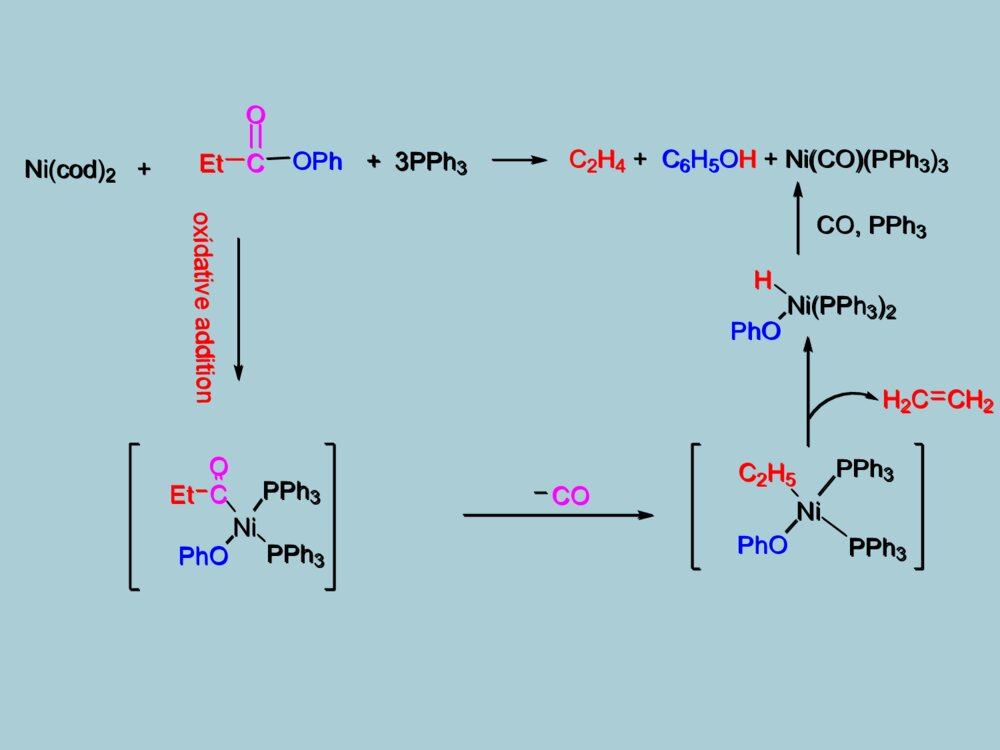
药物合成中的金属有机化学第一章、金属有机化学发展简介第二章、基元反应第三章、人名反应第四章、烯烃复分解反应第五章、Pd催化的碳氢键活化目录第一章、金属有机化学发展简介1760年,Cadet等通过As2O3与CH3COOK反应合成并分离得到人类第一个有机金属化合物[(CH3)2As]2O。1899年,P.Barbier和V.Grignard发明了格氏试剂和格氏反应,并获得了1912年Nobel化学奖。格氏试剂:CH3MgX1951年,P.Pauson和S.A.Miller各自独立合成了二茂铁,这是现代有机金属化学的里程碑。二茂铁:1976年,由于H.C.Brown和G.Witting在有机硼和有机磷化学方面所做的贡献获得Nobel奖。2001~2010年十年间陆续有三次Nobel化学奖授予金属有机化学领域的科学家威廉.诺尔斯野依良治巴里.夏普莱斯2001年不对称催化合成Angew.Chem.Int.Ed.2002,41,1998-2032MeOAcOCOOHNHAcRh(DIPAMP)MeOAcOCOOHNHAcHH3+OMeOAcOCOOHNH2HPPOMeMeODIPAMPL-DOPA金属铑Rh不对称催化合成左旋多巴MeOAcOCOOHNHAcRh(DIPAMP)MeOAcOCOOHNHAcHH3+OMeOAcOCOOHNH2HPPOMeMeODIPAMPL-DOPA伊夫·肖万罗伯特·格拉布理查德·施罗克2005年烯烃复分解反应理查德.赫克根岸英一铃木章2010年钯催化交叉偶联反应NHOOMeAcOOACNHCOCF3EtOEtBenzene-CH=CH2MocatHNEtOEtOOMeOAcOACNHCOCF3HNEtOEtOOOHOHN2HOHFluvirucin-B1A.F.Houri,Z.M.Xu,A.H.Hoveyda,J.Am.Chem.Soc.1995,117,2943.SynthesisofFluvirucin-B1金属钼催化烯烃复分解反应NHOOMeAcOOACNHCOCF3EtOEtBenzene-CH=CH2MocatHNEtOEtOOMeOAcOACNHCOCF3HNEtOEtOOOHOHN2HOHFluvirucin-B1RM+XR\'PdCat.thepreviousreactionsRR\'M=Sn,Li,Cu,etcB(OH)2+CO2MeBrPd(PPh3)4,PhHNa2CO3,6hSuzukireactionCO2Me+XR\'R-BY2PdCat.baseSuzukireactionRR\'Miyaura,N.;Yang,T.;Suzuki,A.Synth.Commun.1981,11,513.2010Nobellecture(Suzukireaction)金属钯催化交叉偶联反应RM+XR\'PdCat.thepreviousreactionsRR\'M=Sn,Li,Cu,etcB(OH)2+CO2MeBrPd(PPh3)4,PhHNa2CO3,6hSuzukireactionCO2Me+XR\'R-BY2PdCat.baseSuzukireactionRR\'第二章、金属有机化学中的基元反应1、氧化加成反应(Oxidativeaddition)+RXMgRMgX0价+2价+1价+3价BLn+LmMAMB第一类A-B:RSX,R-X,RCN,Ar-H例如:+R-XIrXRCOLXLIrCOLXLX价X+2价+RXMgRMgXBLn+LmMAMB+R-XIrXRCOLXLIrCOLXLNi(cod)2++3PPh3C2H4+C6H5OH+Ni(CO)(PPh3)3CEtONiPhOPPh3PPh3H2CCH2Ni(PPh3)2CO,PPh3EtCOOPhHPhOC2H5PhONiPPh3PPh3COoxidativeadditionNi(cod)2++3PPh3C2H4+C6H5OH+Ni(CO)(PPh3)3CEtONiPhOPPh3PPh3H2CCH2Ni(PPh3)2CO,PPh3EtCOOPhHPhOC2H5PhONiPPh3PPh3COoxidativeadditionAAA\'LnLmA\'+MM第二类:金属环化(cyclometalation)H2CCH2MM+2M+MA-A’:RCH=CHR,RN=NR,CS2例如:AAA\'LnLmA\'+MMH2CCH2MM+2M+MNegishi,E.;Ma,S.;Sugihara,T.;Noda,Y.J.Org.Chem.1997,62,1922Cp2Zr(n-Bu)2ZrCp2H-bulneseneHZrn~Bun~BuCpCpHoxidativeadditionCp2Zr(n-Bu)2ZrCp2H-bulneseneHZrn~Bun~BuCpCpHoxidativeaddition2、还原消除反应(ReductiveElimination)LnM+A-BLnMABReverseProcesstothatofO.A1)ElectronwithdrawingOlefin(吸电子烯烃)2)π-Acid(CO)CO是一种常见的π-酸,具有较强的吸电子效应,同样能促进还原消除反应(biPy)NibiPy:联吡啶RR+CN(biPy)NiRRNCR-R+(biPy)Ni(CH2=CHCN)促进还原消除反应的影响因素:LnM+A-BLnMAB(biPy)NibiPy:联吡啶RR+CN(biPy)NiRRNCR-R+(biPy)Ni(CH2=CHCN)不反应C6H6Pt(PEt3)2Ph2I2MeOHPt(PEt3)2PhI[Pt(PEt3)2Ph2I(MeOH)]+I-PhI[Pt(PEt3)2Ph(MeOH)]+I-4)、Cation(正离子效应)3)、加热加热也能促进还原消除反应。不反应C6H6Pt(PEt3)2Ph2I2MeOHPt(PEt3)2PhI[Pt(PEt3)2Ph2I(MeOH)]+I-PhI[Pt(PEt3)2Ph(MeOH)]+I-还原消除反应的立体要求PPdPCH3CH3PhPhPhPh80oCDMSOPPhPhPPhPh80oCDMSO不反应PdH3CCH3CH3-CH3脱去基团必须在同侧才能发生反应PPdPCH3CH3PhPhPhPh80oCDMSOPPhPhPPhPh80oCDMSO不反应PdH3CCH3CH3-CH3M-RM-A-B-R:A=B,C=C,C=N-R,CO等ABBAM-A-RB3、插入反应和反插入反应(Insertion)(a)CO插入到M-R键中,生成酰基金属化合物(途径(I))。(b)和金属以δ键相连的R基团移位到CO上(途径(II))。LnMLnM(途径I)(途径II)RCORCOM-RM-A-B-R:A=B,C=C,C=N-R,CO等ABBAM-A-RBLnMLnM(途径I)(途径II)RCORCOLnMLnMLnM-C-RORCORCOM-H+OCOMOCOH例如:(1)CO的插入M-H+OCOMOCOH(2)CO2的插入甲酸盐型金属羧酸型LnMLnMLnM-C-RORCORCOM-H+OCOMOCOHM-H+OCOMOCOHCO插入两种不同机理:OCCO13COOCCOCOOCCOCOOCCOCH3OC13COOCCOCH31-13CO-CO甲基迁移机理-13COOCCOCH3OCCOCO+OC13COOCCOCO+OCCO13COCH3COCO+CH313COOCCOCO-CO-CO1:2cis-isomer:1trans-isomerOCCOCOOCCOCH3:3CH3COCH3COMn13COMnMnMnMnMnMnMnCO插入机理OCCO13COOCCOCOOCCOCOOCCOCH3OC13COOCCOCH31-13CO-CO甲基迁移机理-13COOCCOCH3OCCOCO+OC13COOCCOCO+OCCO13COCH3COCO+CH313COOCCOCO-CO-CO1:2cis-isomer:1trans-isomerOCCOCOOCCOCH3:3CH3COCH3COMn13COMnMnMnMnMnMnMnCO插入机理L2NiR\'X+R-MgXL2NiR\'R+MgX2KumadaReactionPhBrPhBr+Me-MgBrMe-MgBr+PhMePhMe4、金属交换反应(Transmentallation)R-X+MR-MXR\'-M\'R-M-R\'MR-R\'O.ATransmentallationRed.elim.构型不变L2NiR\'X+R-MgXL2NiR\'R+MgX2KumadaReactionPhBrPhBr+Me-MgBrMe-MgBr+PhMePhMeR-X+MR-MXR\'-M\'R-M-R\'MR-R\'O.ATransmentallationRed.elim.5、其他反映类型β-H攫取反应-H-OPhCH2=CH-OPhCH2=CH2PPh3BrPh3PCH2CH2OPhPdCp2ZrBu2+RRCp2ZrRZrCpMeOOMeMeOCp-H-OPhCH2=CH-OPhCH2=CH2PPh3BrPh3PCH2CH2OPhPdCp2ZrBu2+RRCp2ZrRZrCpMeOOMeMeOCp第三章、人名反应Heck反应Negishi反应Suzuki反应Stille反应Sonogashira反应Kumada反应1、Heck反应H++BaseR+Base-HXRXPd0Pd(0)RBaseHH+Base-HX-HeliminationRPdXRXRHPdXPdXRRHPdXHeck反应机理H++BaseR+Base-HXRXPd0Pd(0)RBaseHH+Base-HX-HeliminationRPdXRXRHPdXPdXRRHPdXHeck反应机理Heck反应的立体选择性HMeHPhPd+PhMeHPhHPd+HPhMePhHMePhHPhEisomerSyn-additionrotationeliminationPd+-PhMeHHPh+PdPhHMePhHPd+HMePhPhHPhMeHPhZisomerSyn-additionrotationeliminationPd+-PhHMeHPhPd+PhMeHPhHPd+HPhMePhHMePhHPhEisomerSyn-additionrotationeliminationPd+-PhMeHHPh+PdPhHMePhHPd+HMePhPhHPhMeHPhZisomerSyn-additionrotationeliminationPd+-PhIC6H13+OHC6H13OHC6H13CHOC6H13HaPd+OHHbpathaPd(OAc)2,Ag2CO3Bu4NHSO4,CH3CN54%pathbPd(OAc)2,PPh3AgOAcDMF,61%(1)分子间的Heck反应IC6H13+OHC6H13OHC6H13CHOC6H13HaPd+OHHbpathaPd(OAc)2,Ag2CO3Bu4NHSO4,CH3CN54%pathbPd(OAc)2,PPh3AgOAcDMF,61%NOPhINOPhPdINOPhPdILnHNOPhNOPh(CH2)nNOPh(CH2)n(CH2)n(CH2)nba(CH2)n(CH2)n-Helimination-HeliminationabLnIPdCH3CNn-Bu4NClK2CO3Ph3PPd(OAc)2(2)分子内的Heck反应NOPhINOPhPdINOPhPdILnHNOPhNOPh(CH2)nNOPh(CH2)n(CH2)n(CH2)nba(CH2)n(CH2)n-Helimination-HeliminationabLnIPdCH3CNn-Bu4NClK2CO3Ph3PPd(OAc)2NMeOOHIMeOOCH20%Pd(OCOCF3)2(PPh3)2PhMe,PMPOMeOHNMeOOCLnPdOMeONMeOOCOMeONMeOOCOHMorphineL.EOverman,JACS,1993,11028andTL,1994,3453NMeOOHIMeOOCH20%Pd(OCOCF3)2(PPh3)2PhMe,PMPOMeOHNMeOOCLnPdOMeONMeOOCOMeONMeOOCOHMorphineNHNMeIHHNHNMePdLnIHHNHNMeHHHPdLnINHNMeHHNNMeHHPd(OAc)2(0.5mol%)K2CO3,TBACl,DMF79%HeckReactionVHRawal,JACS,1993,3030;(+)-dehydrotubifolineAConciseSynthesisofDehydrotubifolineNHNMeIHHNHNMePdLnIHHNHNMeHHHPdLnINHNMeHHNNMeHHPd(OAc)2(0.5mol%)K2CO3,TBACl,DMF79%HeckReactionVHRawal,JACS,1993,3030;(+)-dehydrotubifolineAConciseSynthesisofDehydrotubifoline+R\'-ZnXPd0R-R\'R-XR=alkenyl,aryl,allyl,benzyl,propargylR\'=akenyl,aryl,alkynyl,alkyl,benzyl,allyl2、Negishi反应RBrBrZnR\'RR\'+Pd.catAr-X+BrZnRR\'1mol-%Pd(OAc)22mol-%CPhosTHF/toluene(1:0or3:2)0.5-0.6hArRR\'例如:CPhos:Pcy2Me2NNMe2C.Han,S.L.Buchwald,J.Am.Chem.Soc.,2009,131,7532-7533.+R\'-ZnXPd0R-R\'R-XR=alkenyl,aryl,allyl,benzyl,propargylR\'=akenyl,aryl,alkynyl,alkyl,benzyl,allylRBrBrZnR\'RR\'+Pd.catAr-X+BrZnRR\'1mol-%Pd(OAc)22mol-%CPhosTHF/toluene(1:0or3:2)0.5-0.6hArRR\'CPhos:Pcy2Me2NNMe2Negishi反应机理IOTMSTBDMSO1)n-BnLi2)ZnBr2,THF3)Evaporation4)DMFZnLnOTMSTBDMSO5%PdCl2[P(furyl)3]2OTBDMSIOTMSTBDMSOOTBDMSOTMSTBDMSOOHNakienoneTL,1996,4678SynthesisofNakienoneIOTMSTBDMSO1)n-BnLi2)ZnBr2,THF3)Evaporation4)DMFZnLnOTMSTBDMSO5%PdCl2[P(furyl)3]2OTBDMSIOTMSTBDMSOOTBDMSOTMSTBDMSOOHNakienoneIPhPhEtBrZnONMe2+Pd.cat(Z)-TamoxifenONMe2PhPhEtIOOPMPMeMeOTBSMeOOPMPMeMeOTBSMePMBOOTBS1)t-BuLi,-780C,ZnCl22)L4Pd,Et2O,250COPMBMeOTBSIAnew.Chem.Int.Ed.Engl.1997,93JACS,1995,12011IPhPhEtBrZnONMe2+Pd.cat(Z)-TamoxifenONMe2PhPhEtIOOPMPMeMeOTBSMeOOPMPMeMeOTBSMePMBOOTBS1)t-BuLi,-780C,ZnCl22)L4Pd,Et2O,250COPMBMeOTBSI3、Suzuki反应R-X+R\'-BY2Pd0,baseR-RPd(0)RPdOR\'L2RPdXL2R\'\'PdRL2RXR\'\'RR\'\'BR\'O-BR\'ONaNaXSuzuki反应机理:R-X+R\'-BY2Pd0,baseR-RPd(0)RPdOR\'L2RPdXL2R\'\'PdRL2RXR\'\'RR\'\'BR\'O-BR\'ONaNaX+BrPh1%PdCl22%PPh3NaOEt/EtOHinbenzenen-BuPhn-BuBOOL2ROPdPh+122R\'OBOOPdPhn-BuLL3ASuzukiChem.Commun,1979,866+BrPh1%PdCl22%PPh3NaOEt/EtOHinbenzenen-BuPhn-BuBOOL2ROPdPh+122R\'OBOOPdPhn-BuLL3pinacolboranePd(PPh3)2Cl2TEAinTHFPMBOPMBOOBMPBrPMBOPMBOBOBMPOOOOOMeOIPd(dppf)2Cl2aq.NaCO3,rt89%OOOMeOOBMPOBMPPMBOOOOMeOOHOHOHirellanineATL,2010,2883SynthesisofHirellaninepinacolboranePd(PPh3)2Cl2TEAinTHFPMBOPMBOOBMPBrPMBOPMBOBOBMPOOOOOMeOIPd(dppf)2Cl2aq.NaCO3,rt89%OOOMeOOBMPOBMPPMBOOOOMeOOHOHOHirellanineA4、Stille反应R-X+R\'-SnPd.catR-R\'Pd(0)L2RPdLLXRPdLLR\'PdR\'LRL213RXRR\'R\'SnR\'\'3XSnR\'\'3OxidativeadditionReductiveeliminationStille反应机理:R-X+R\'-SnPd.catR-R\'Pd(0)L2RPdLLXRPdLLR\'PdR\'LRL213RXRR\'R\'SnR\'\'3XSnR\'\'3OxidativeadditionReductiveeliminationTIPSOMeOMeOOMeMeRBu3SnOOSBTTBSOONOOOOTESMeI[(2-furyl)3P]2PdCl2DME/THFTIPSOMeOMeOOMeMeROOSBTTBSOONOOOOTESMeJACS,1991,9585TIPSOMeOMeOOMeMeRBu3SnOOSBTTBSOONOOOOTESMeI[(2-furyl)3P]2PdCl2DME/THFTIPSOMeOMeOOMeMeROOSBTTBSOONOOOOTESMeoNHNoOTBSOOHBu3SnOMeIMeOoNHNoOTBSOOHMeOPd2(dba)3CHCl3(64mol%)Ph3As(2.56eq.)DIPEA(41.6eq.)inDMF,23C,48hOMe72%yieldTAS-FinDMF/EtOAc,230C,2hoNHNoOTBSOOHMeOOMe80%yieldLejimalideBanticanceractivityJOC,2011,5157oNHNoOTBSOOHBu3SnOMeIMeOoNHNoOTBSOOHMeOPd2(dba)3CHCl3(64mol%)Ph3As(2.56eq.)DIPEA(41.6eq.)inDMF,23C,48hOMe72%yieldTAS-FinDMF/EtOAc,230C,2hoNHNoOTBSOOHMeOOMe80%yieldLejimalideBanticanceractivity5、Sonogashira反应R-X+R\'Pd0,CuX,baseRR\'RCuI,R\'\'3NRCuR\'XR\'PdXRPdR\'RR\'Pd(0)OTsRR\'3eq.+5mol%PdCl2(CH3CN)24.5eq.CuIEtCNreflux,>10hRR\'ligandPCy2Angew.Chem.,2003,115,6175反应机理:R-X+R\'Pd0,CuX,baseRR\'RCuI,R\'\'3NRCuR\'XR\'PdXRPdR\'RR\'Pd(0)OTsRR\'3eq.+5mol%PdCl2(CH3CN)24.5eq.CuIEtCNreflux,>10hRR\'ligandPCy2Angew.Chem.,2003,115,6175O+R\'OCHOR\'3.85eq.NPN32.48eq.NfF,1.5eq.LiClDMF,0oC-r.t.,17-24hNf:-SO2(CF2)3CF3ONf+R\'R\'nRn=1,2,3orRn5mol%Pd(OAc)20.1eq.PPh30.1eq.CuIiPr2NH/DMF(3:2)r.t.or60oC,4-24hAngew.Chem.Int.Ed.,2006,45,4019RClICO2EtR\'RClCO2EtR\'+3eq.0.1eq.Pd(PPh3)2Cl20.15eq.CuI3eq.DIPEAdioxane,r.t.,2hAr+RIR\'RR\'Ar10mol%[Cu(bipy)PPh3Br]2eq.K2CO3toluene,110oC,8hJ.Org.Chem.,2006,71,3615Org.Lett.,2004,6,1441O+R\'OCHOR\'3.85eq.NPN32.48eq.NfF,1.5eq.LiClDMF,0oC-r.t.,17-24hNf:-SO2(CF2)3CF3ONf+R\'R\'nRn=1,2,3orRn5mol%Pd(OAc)20.1eq.PPh30.1eq.CuIiPr2NH/DMF(3:2)r.t.or60oC,4-24hAngew.Chem.Int.Ed.,2006,45,4019RClICO2EtR\'RClCO2EtR\'+3eq.0.1eq.Pd(PPh3)2Cl20.15eq.CuI3eq.DIPEAdioxane,r.t.,2hAr+RIR\'RR\'Ar10mol%[Cu(bipy)PPh3Br]2eq.K2CO3toluene,110oC,8hJ.Org.Chem.,2006,71,3615Org.Lett.,2004,6,1441NINNONHFmocOPdH+BocHNCO2BnZnIPd2dba3(10mol%)P(o-tol)3(1.0eq.)CuBr.DMS(5mol%)inDMA,25-380C,2hNNNONHFmocOPdHBocHNCO2BnNNNONHOPdHNHNH2ONHOOKapakahineFCytotoxicityOL,2010,2154NINNONHFmocOPdH+BocHNCO2BnZnIPd2dba3(10mol%)P(o-tol)3(1.0eq.)CuBr.DMS(5mol%)inDMA,25-380C,2hNNNONHFmocOPdHBocHNCO2BnNNNONHOPdHNHNH2ONHOOKapakahineFCytotoxicityOCO2MeOHOMe+MeOTBS1)LDA(2.0eq.)ZnBr(3.0eq.)inTHF,-780Cto00CPdCl2(dppf)(8.0mol%)inTHF00Cto500C,4h2)TBAFOCO2MeOHOMeMeOHOCO2MeMeMeOO(-)-Z-deoxypukalideanti-infiammatoryAagew.Chem.Int.Ed.2008,7314OCO2MeOHOMe+MeOTBS1)LDA(2.0eq.)ZnBr(3.0eq.)inTHF,-780Cto00CPdCl2(dppf)(8.0mol%)inTHF00Cto500C,4h2)TBAFOCO2MeOHOMeMeOHOCO2MeMeMeOO(-)-Z-deoxypukalideanti-infiammatory6、Kumada反应+R-MgXNi,catR\'-XR\'-R+MgX2NiL2NiLArRLNiArLRLNiArLBrLAr-RAr-BrR-MgBrMgBr2反应机理:+R-MgXNi,catR\'-XR\'-R+MgX2NiL2NiLArRLNiArLRLNiArLBrLAr-RAr-BrR-MgBrMgBr2OMeOMeOMgCl+OOClOOMeOMeOMeOTEA/cat.(dppe)NiCl2OOMeOMeHNNH2OHOH2NOAliskirenJohnsonandLee(2010).ModernDrugSynthesis.Hoboken,NJ:JohnWiley&Sons,Inc.pp.153–154.SynthesisofAliskirenOMeOMeOMgCl+OOClOOMeOMeOMeOTEA/cat.(dppe)NiCl2OOMeOMeHNNH2OHOH2NOAliskirenGeneralMechanismsofPd-CatalyzedCross-CouplingReactionLnPd+Ar-XaLmPdArXHArPdXLmPdArAr\'ArbasesaltAr-Ar\'\'Ar\'-MM-XHbcdeHeckSuzuki(B)Negishi(Zn)Stille(Sn)Sonogashiraa.Oxidativeadditionb.ReductiveEliminationc.Insertiond.TransmentallationLnPd+Ar-XaLmPdArXHArPdXLmPdArAr\'ArbasesaltAr-Ar\'\'Ar\'-MM-XHbcdeHeckSuzuki(B)Negishi(Zn)Stille(Sn)Sonogashira第四章、烯烃复分解反应(OlefinMetathesis)R1R1R2R2R1R1R2R2+R1R1R1R1R2R2R2R2+催化剂在金属催化剂的作用下,两分子的烯烃交换与双键相连的碳原子,而形成新的烯烃分子。R1R2+[M]R1+R2金属卡宾化合物生成的烯烃是Z形和E形的混合物双键在碳链的末端,生成一乙烯分子和另两端结合的大分子R1R1R2R2R1R1R2R2+R1R1R1R1R2R2R2R2+催化剂R1R2+[M]R1+R2卡宾催化剂的应用RuClNPhMesNNMesGrubbsIIcatalystClRuClPCy3PhHMesNNMesClRuClOMesNNMesClRuClNPhHMesNNMesi-prMeNCl1234CNOCNPhOHOnCNHOCNHOCNnCNOHOHOCNnMeCNn1+CuCl1+CuCl1+CuCl344+Ti(Oi-Pr)4n=1,80%n=2,92%n=1,56%n=2,61%1+CuClRuClNPhMesNNMesGrubbsIIcatalystClRuClPCy3PhHMesNNMesClRuClOMesNNMesClRuClNPhHMesNNMesi-prMeNCl1234CNOCNPhOHOnCNHOCNHOCNnCNOHOHOCNnMeCNn1+CuCl1+CuCl1+CuCl344+Ti(Oi-Pr)4n=1,80%n=2,92%n=1,56%n=2,61%1+CuCl三、反应历程R1R2+R1R2++nn+nCMRCMROMPADMET交错复分解关环复分解开环复分解聚合非环复分解聚合分解反应的类型R1R2+R1R2++nn+nCMRCMROMPADMETONNOOOSOOBrHNOOOONNOOOSOOBrHNOOORutolueneONNOOOHNOOONNSNOMeBoehringerIngelheimHepatitisCDrugsT.Nicola,M.Brenner.K.Donsbach.OrganicProcessandDevelopment,25,27.ONNOOOSOOBrHNOOOONNOOOSOOBrHNOOORutolueneONNOOOHNOOONNSNOMeTBSOMeCHOMeBPlnOTBDPSCO2MeTBSOMeMeOHOTBDPSOTBSOMeMeOHOTBSOMeMeOHOTBSOMeMeOHOHOHMeMeOHOHOHOPDC,TMSCIDCM.0OC,4hGrubbsII(5mol%)DCM,12h93%D.G.Hall,etal,J.AM.Chem.Soc.2010,132,1488TotalSynthesisOf(+)-ChinensioideBTBSOMeCHOMeBPlnOTBDPSCO2MeTBSOMeMeOHOTBDPSOTBSOMeMeOHOTBSOMeMeOHOTBSOMeMeOHOHOHMeMeOHOHOHOPDC,TMSCIDCM.0OC,4hGrubbsII(5mol%)DCM,12h93%OPMBHOMeTBSOMeOOMeMeOGrubbsII90%TBSOMeOOMeOTBSOMeOOMeBu3SnOOMeMeOMeMeMeOMeOTBSOTBSOIMeOSBTMeOHOMeMeHOMeOMeOHOHOMeOSBTMeOHMeOOMeEtnangien(1)+DirkMenche,etal,J.Am.Chem.Soc.2009,131,11678OPMBHOMeTBSOMeOOMeMeOGrubbsII90%TBSOMeOOMeOTBSOMeOOMeBu3SnOOMeMeOMeMeMeOMeOTBSOTBSOIMeOSBTMeOHOMeMeHOMeOMeOHOHOMeOSBTMeOHMeOOMeEtnangien(1)+《Palladium-CatalyzedTransformationsofAlkylC−HBonds》Jin-QuanYuDOI:10.1021/acs.chemrev.6b00622Chem.Rev.2017,117,8754−8786JianHe,MasayukiWasa,KelvinS.L.Chan,QianShao,andJin-QuanYu*+COCO2Hcat.Pd(OAc)2Cu(OAc)2K2S2O8TFA,800C4.3%Yieldbasedon1MeMe+COcat.Pd(O2CEt)2CuSO4K2S2O8TFA,800CMeMeMeCO2HCO2H+360%yieldbasedonPd300%yieldbasedonPd1Fujiwaraetal,.1989add19932Pd(II)-CatalyzedCarbonylationofAlkanes1、C(sp3)-HActivationofAlkanesCH4CF2CO2CH3cat.5K2S2O8TFA,TFAA900C3000%yieldbasedon2Strassneretal.,2002NPdNNNMeMeBrBr2+COCO2Hcat.Pd(OAc)2Cu(OAc)2K2S2O8TFA,800C4.3%Yieldbasedon1MeMe+COcat.Pd(O2CEt)2CuSO4K2S2O8TFA,800CMeMeMeCO2HCO2H+360%yieldbasedonPd300%yieldbasedonPd1Fujiwaraetal,.1989add19932CH4CF2CO2CH3cat.5K2S2O8TFA,TFAA900C3000%yieldbasedon2Strassneretal.,2002NPdNNNMeMeBrBr22、C(SP3)−HActivationDirectedByC-XBonds(VIAPd(0)/Pd(II)Catalysis)Dyker’sSynthesisof1,2-DihydrocyclobutabenzeneIMeMeHPdIIMeMeHIPd0-HXPdIIMeMePdIVMeMePdIIMeMeArBrArPdIIBrArPdIIXMeMeHArPdIIMeMeArMeMecat.Pd(OAc)2Ar-BrK2CO3,n-Bu4NBrDMF,1050CAr-BrArPdII-Br-Pd0-HX-Pd0IMeMeHPdIIMeMeHIPd0-HXPdIIMeMePdIVMeMePdIIMeMeArBrArPdIIBrArPdIIXMeMeHArPdIIMeMeArMeMecat.Pd(OAc)2Ar-BrK2CO3,n-Bu4NBrDMF,1050CAr-BrArPdII-Br-Pd0-HX-Pd03、C(SP3)−HActivationDirectionbystronglyCoordinationAuxiliariesMeNHOMeMeHMeNHOMeMePd2Na2PdCl4(1equiv)NaOAc,MeOHShawetal.1978ReactionsofOxime-DerivedPalladacyclesMeNHOMeMeClMeNHOMeMePd21)Cl2,CCl42)NaBH3CNNaBD3CNMeOH,THFMeNHOMeMeD64%41%MeNHOMeMePdNCl1)Pb(OAc)42)NaBH4MeNHOMeMeOAcpyridineShawetBaldwinetal,1985NNHNNPdOAcPd(OAc)2(1equiv)AcOH,1000CHirakietal.1984MeNHOMeMeHMeNHOMeMePd2Na2PdCl4(1equiv)NaOAc,MeOHShawetal.1978ReactionsofOxime-DerivedPalladacyclesMeNHOMeMeClMeNHOMeMePd21)Cl2,CCl42)NaBH3CNNaBD3CNMeOH,THFMeNHOMeMeD64%41%MeNHOMeMePdNCl1)Pb(OAc)42)NaBH4MeNHOMeMeOAcpyridineShawetBaldwinetal,1985NNHNNPdOAcPd(OAc)2(1equiv)AcOH,1000CHirakietal.19844、Pd-CatalyzedC(sp3)−HHalogenationNONHHRNONHFRcat.Pd(OAc)2,NFSIAg2O,PivOHchlorobenzene90-1200C33-77%Xuetal.2015NONHHR\'R/HNONHXR\'R/Hcat.Pd(OAc)2NXS,AcOH,rtX=Cl,Br,IRaoetal.2016NHNHOMeMeMeMeNNOMeMeMeMecat.Pd(OAc)2PhI(OAc)2toluene,800CDaugulisetal.201288%NONHHRNONHFRcat.Pd(OAc)2,NFSIAg2O,PivOHchlorobenzene90-1200C33-77%Xuetal.2015NONHHR\'R/HNONHXR\'R/Hcat.Pd(OAc)2NXS,AcOH,rtX=Cl,Br,IRaoetal.2016NHNHOMeMeMeMeNNOMeMeMeMecat.Pd(OAc)2PhI(OAc)2toluene,800CDaugulisetal.201288%MeSNOMeO2CHH+OMeOMeOMeIcat.Pd(OAc)2Ag2CO3PivOHHFIP,900CMeSNOMeO2CHOMeOMeOMe52%LiOt-BuMeSNOCO2MeHOMeOMeOMe79%,d.r.>10:1MeSNOCO2MeOMeOMeOMeMeOMeO46%cat.Pd(OAc)2Ag2CO3t-BuOH,75%OMeOMeINOOMeOMeOMeMeOMeOONOOpiperaborenineBGutekunst,W.R.;Baran,P.S.J.Am.Chem.Soc.2011,133,19076−19079.MeSNOMeO2CHH+OMeOMeOMeIcat.Pd(OAc)2Ag2CO3PivOHHFIP,900CMeSNOMeO2CHOMeOMeOMe52%LiOt-BuMeSNOCO2MeHOMeOMeOMe79%,d.r.>10:1MeSNOCO2MeOMeOMeOMeMeOMeO46%cat.Pd(OAc)2Ag2CO3t-BuOH,75%OMeOMeINOOMeOMeOMeMeOMeOONOOpiperaborenineB5、AminoAcid-DirectedC(sp3)−HArylationHNHOHOOPhthNArNHOHOOPhthNHNOOOPhthNPdOAcvia:cat.Pd(OAc)2Ar-I,AgOAcKF,HEIP1000C,20h69-92%Yuetal.2014Yuetal.2016HR2OR1ArR2OR1HR2R1NPdOOOAcvia;39-85%cat.Pd(OAc)2glycine.AgTFAHFIP:AcOH(3:1)Ar-I,1100C,36hHHROArHROHNRPdOOR\'OAc54-88%90-96%eecat.Pd(OAc)2cat.L-tert-leucineAr-I,1100C,36hGeetal.2016HMeOHHMeOPhcat.Pd(OAc)2NH2(CH2)2CO2HPh-I,AgTFAHEIP:AcOH(5:1)800C,24h,N271%via:HNHOHOOPhthNArNHOHOOPhthNHNOOOPhthNPdOAcvia:cat.Pd(OAc)2Ar-I,AgOAcKF,HEIP1000C,20h69-92%Yuetal.2014Yuetal.2016HR2OR1ArR2OR1HR2R1NPdOOOAcvia;39-85%cat.Pd(OAc)2glycine.AgTFAHFIP:AcOH(3:1)Ar-I,1100C,36hHHROArHROHNRPdOOR\'OAc54-88%90-96%eecat.Pd(OAc)2cat.L-tert-leucineAr-I,1100C,36hGeetal.2016HMeOHHMeOPhcat.Pd(OAc)2NH2(CH2)2CO2HPh-I,AgTFAHEIP:AcOH(5:1)800C,24h,N271%via:6、C(SP3)−HActivationDirectionbyweeklyCoordinationAuxiliaries:HOHOMeMecat.Pd(OAc)2K2HPO4,BQAgCO3,t-BuOH1000CBOOPhMeMePd-LOOKMeMePhPhOHOMeMe38%HOHOMeHcat.Pd(OAc)2Ph-BXnK2HPO4,[O]1000COOKMeHPdLnPhOHOMeH0%HOOHRR\'Pd(OAc)2Pd(OAc)2MOAcHOORR\'Pd(II)noC-HactivationHOORR\'MM=Na,K,etcPdAcOOAcOORR\'MPdLOAcLHOAcWang,D.-H.;Giri,R.;Yu,J.-Q.J.Am.Chem.Soc.2008,130,7190−7191.HOHOMeMecat.Pd(OAc)2K2HPO4,BQAgCO3,t-BuOH1000CBOOPhMeMePd-LOOKMeMePhPhOHOMeMe38%HOHOMeHcat.Pd(OAc)2Ph-BXnK2HPO4,[O]1000COOKMeHPdLnPhOHOMeH0%HOOHRR\'Pd(OAc)2Pd(OAc)2MOAcHOORR\'Pd(II)noC-HactivationHOORR\'MM=Na,K,etcPdAcOOAcOORR\'MPdLOAcLHOAcSynthesisofTeleocidinB-4CoreOMeHMeMeNOMeOMeOMeMeMeNOMeOMeNaOAcAcOHPdClMePrB(OH)2Ag2O,DMF75%OMeMeMeNOMeOMeMei-PrOMeNOMeOMeDCMMeSO3HPrMei-i-MeHPdCl2PdCl2NaOAc86%83%OMeNOMeOMePdClMePrMe1)CO(40atm)MeOH2)SiO2CHCl3NHMeMePri-OOHNHMeMePri-OOH56%9%+OMeHMeMeNOMeOMeOMeMeMeNOMeOMeNaOAcAcOHPdClMePrB(OH)2Ag2O,DMF75%OMeMeMeNOMeOMeMei-PrOMeNOMeOMeDCMMeSO3HPrMei-i-MeHPdCl2PdCl2NaOAc86%83%OMeNOMeOMePdClMePrMe1)CO(40atm)MeOH2)SiO2CHCl3NHMeMePri-OOHNHMeMePri-OOH56%9%+



















