








《高中化学 末整合提升物质及其变化PPT课件下载》是由用户上传到老师板报网,本为文库资料,大小为864.82 KB,总共有33页,格式为pptx。授权方式为VIP用户下载,成为老师板报网VIP用户马上下载此课件。文件完整,下载后可编辑修改。
- 文库资料
- 33页
- 864.82 KB
- VIP模板
- pptx
- 数字产品不支持退货
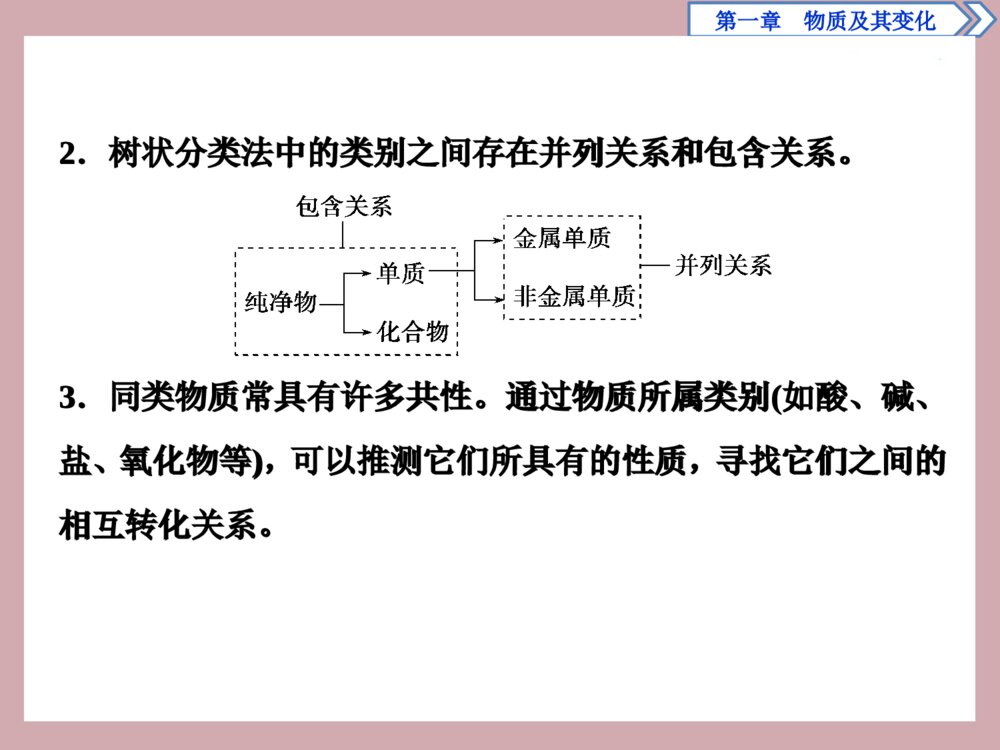
章末整合提升第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/一、物质常见的分类方法及其应用1.交叉分类法涉及的不同类别之间是并列与交叉关系。第一章 物质及其变化一、物质常见的分类方法及其应用1.交叉分类法涉及的不同类别之间是并列与交叉关系。第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/2.树状分类法中的类别之间存在并列关系和包含关系。3.同类物质常具有许多共性。通过物质所属类别(如酸、碱、盐、氧化物等),可以推测它们所具有的性质,寻找它们之间的相互转化关系。2.树状分类法中的类别之间存在并列关系和包含关系。3.同类物质常具有许多共性。通过物质所属类别(如酸、碱、盐、氧化物等),可以推测它们所具有的性质,寻找它们之间的相互转化关系。第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/【针对训练】1.化学概念在逻辑上存在如下关系:下列说法正确的是()A.化合物与纯净物属于重叠关系(如图④)B.化合物与碱性氧化物属于交叉关系(如图③)C.分解反应与复分解反应属于并列关系(如图②)D.钠盐与碳酸盐属于并列关系(如图②)【针对训练】1.化学概念在逻辑上存在如下关系:下列说法正确的是()A.化合物与纯净物属于重叠关系(如图④)B.化合物与碱性氧化物属于交叉关系(如图③)C.分解反应与复分解反应属于并列关系(如图②)D.钠盐与碳酸盐属于并列关系(如图②)第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/解析:选C。A项,纯净物包含化合物,属于包含关系;B项,碱性氧化物是化合物中的一种,属于包含关系;D项,钠盐与碳酸盐属于交叉关系。解析:选C。A项,纯净物包含化合物,属于包含关系;B项,碱性氧化物是化合物中的一种,属于包含关系;D项,钠盐与碳酸盐属于交叉关系。第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/2.按照物质的树状分类法和交叉分类法,对于HCl的分类正确的是()①酸②强酸③一元酸④多元酸⑤含氧酸⑥无氧酸⑦化合物⑧混合物A.全部B.①②④⑤⑧C.①②③⑥⑦D.①③④⑤⑥2.按照物质的树状分类法和交叉分类法,对于HCl的分类正确的是()①酸②强酸③一元酸④多元酸⑤含氧酸⑥无氧酸⑦化合物⑧混合物A.全部B.①②④⑤⑧C.①②③⑥⑦D.①③④⑤⑥第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/解析:选C。HCl电离时生成的阳离子全部是氢离子,从组成上分类属于酸,故①正确;HCl完全电离,是强酸,故②正确;1molHCl可电离出1molH+,属于一元酸,故③正确,④错误;HCl不含氧元素,不属于含氧酸,故⑤错误;HCl属于无氧酸,故⑥正确;HCl由两种元素组成,属于化合物,故⑦正确;HCl是由两种元素组成的化合物,是纯净物,不是混合物,故⑧错误;综上所述,①②③⑥⑦正确,故选C。解析:选C。HCl电离时生成的阳离子全部是氢离子,从组成上分类属于酸,故①正确;HCl完全电离,是强酸,故②正确;1molHCl可电离出1molH+,属于一元酸,故③正确,④错误;HCl不含氧元素,不属于含氧酸,故⑤错误;HCl属于无氧酸,故⑥正确;HCl由两种元素组成,属于化合物,故⑦正确;HCl是由两种元素组成的化合物,是纯净物,不是混合物,故⑧错误;综上所述,①②③⑥⑦正确,故选C。第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/二、离子方程式的正误判断及离子共存1.离子方程式的书写要点(1)易溶、易电离的物质(包括强酸、强碱、大多数可溶性盐)以实际参加反应的离子符号表示。非电解质、难电离的物质(包括弱酸、弱碱、水等)、难溶物、单质、气体、氧化物等用化学式表示。(2)离子方程式两边的原子个数相等,电荷总数相等。(3)检查各项是否都有公约数,检查是否漏写必要的反应条件。二、离子方程式的正误判断及离子共存1.离子方程式的书写要点(1)易溶、易电离的物质(包括强酸、强碱、大多数可溶性盐)以实际参加反应的离子符号表示。非电解质、难电离的物质(包括弱酸、弱碱、水等)、难溶物、单质、气体、氧化物等用化学式表示。(2)离子方程式两边的原子个数相等,电荷总数相等。(3)检查各项是否都有公约数,检查是否漏写必要的反应条件。第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/2.离子方程式的正误判断(1)看离子反应是否符合客观事实,如Fe跟稀H2SO4反应,写成2Fe+6H+===2Fe3++3H2↑是错误的。(2)看化学式拆写是否正确,如NaHCO3与稀盐酸反应写成2H++CO2-3===CO2↑+H2O是错误的,因为HCO-3是弱酸的酸根,不能拆开。(3)看离子方程式两边原子、电荷是否相等,如Zn+Ag+===Zn2++Ag两边正电荷不相等。(4)看是否漏掉部分离子反应,如H2SO4溶液与Ba(OH)2溶液的反应,写成Ba2++SO2-4===BaSO4↓或H++OH-===H2O都是错误的。2.离子方程式的正误判断(1)看离子反应是否符合客观事实,如Fe跟稀H2SO4反应,写成2Fe+6H+===2Fe3++3H2↑是错误的。(2)看化学式拆写是否正确,如NaHCO3与稀盐酸反应写成2H++CO2-3===CO2↑+H2O是错误的,因为HCO-3是弱酸的酸根,不能拆开。(3)看离子方程式两边原子、电荷是否相等,如Zn+Ag+===Zn2++Ag两边正电荷不相等。(4)看是否漏掉部分离子反应,如H2SO4溶液与Ba(OH)2溶液的反应,写成Ba2++SO2-4===BaSO4↓或H++OH-===H2O都是错误的。第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/3.判断离子共存(1)离子间因生成难溶于水(或微溶于水)的物质而不能大量共存①OH-分别与Fe2+、Fe3+、Mg2+、Cu2+、Al3+、Ag+、Ca2+在溶液中不能大量共存;②Ba2+(或Ca2+)分别与SO2-4、SO2-3、CO2-3、SiO2-3、PO3-4在溶液中不能大量共存;③Ag+分别与Cl-、Br-、I-、CO2-3、SO2-4等在溶液中不能大量共存;④Cu2+(或Fe2+)分别与S2-在溶液中不能大量共存。3.判断离子共存(1)离子间因生成难溶于水(或微溶于水)的物质而不能大量共存①OH-分别与Fe2+、Fe3+、Mg2+、Cu2+、Al3+、Ag+、Ca2+在溶液中不能大量共存;②Ba2+(或Ca2+)分别与SO2-4、SO2-3、CO2-3、SiO2-3、PO3-4在溶液中不能大量共存;③Ag+分别与Cl-、Br-、I-、CO2-3、SO2-4等在溶液中不能大量共存;④Cu2+(或Fe2+)分别与S2-在溶液中不能大量共存。第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/(2)离子间因生成气体而不能大量共存H+分别与CO2-3、HCO-3、S2-、HS-、SO2-3、HSO-3在溶液中不能大量共存。(3)多元弱酸的酸式酸根(如HCO-3、HSO-3、HS-等)在强酸或强碱性溶液中均不能大量共存例如:HCO-3+H+===CO2↑+H2O;HCO-3+OH-===CO2-3+H2O。(4)离子间因发生氧化还原反应而不能大量共存例如:S2-、I-、Fe2+等还原性离子与NO-3(H+)、MnO-4(H+)、ClO-等氧化性离子在溶液中不能大量共存。(2)离子间因生成气体而不能大量共存H+分别与CO2-3、HCO-3、S2-、HS-、SO2-3、HSO-3在溶液中不能大量共存。(3)多元弱酸的酸式酸根(如HCO-3、HSO-3、HS-等)在强酸或强碱性溶液中均不能大量共存例如:HCO-3+H+===CO2↑+H2O;HCO-3+OH-===CO2-3+H2O。(4)离子间因发生氧化还原反应而不能大量共存例如:S2-、I-、Fe2+等还原性离子与NO-3(H+)、MnO-4(H+)、ClO-等氧化性离子在溶液中不能大量共存。第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/【针对训练】3.判断下列离子方程式是否正确,错误的说明原因,并写出正确的离子方程式。(1)铁粉溶于稀硫酸2Fe+6H+===2Fe3++3H2↑(2)用氯化铁溶液制取Fe(OH)3胶体Fe3++3H2O===Fe(OH)3↓+3H+(3)氢氧化铜溶于盐酸OH-+H+===H2O(4)氢氧化钡溶液与硫酸铜溶液混合2OH-+Cu2+===Cu(OH)2↓【针对训练】3.判断下列离子方程式是否正确,错误的说明原因,并写出正确的离子方程式。(1)铁粉溶于稀硫酸2Fe+6H+===2Fe3++3H2↑(2)用氯化铁溶液制取Fe(OH)3胶体Fe3++3H2O===Fe(OH)3↓+3H+(3)氢氧化铜溶于盐酸OH-+H+===H2O(4)氢氧化钡溶液与硫酸铜溶液混合2OH-+Cu2+===Cu(OH)2↓第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/(5)铝与氯化铜溶液发生置换反应Al+Cu2+===Al3++Cu(6)硫酸溶液与氢氧化钡溶液混合H++OH-+SO2-4+Ba2+===BaSO4↓+H2O(7)碳酸钙溶于硝酸溶液CaCO3+2H+===Ca2++CO2↑+H2O(8)碳酸氢钙溶液与足量氢氧化钠溶液混合HCO-3+OH-===CO2-3+H2O(5)铝与氯化铜溶液发生置换反应Al+Cu2+===Al3++Cu(6)硫酸溶液与氢氧化钡溶液混合H++OH-+SO2-4+Ba2+===BaSO4↓+H2O(7)碳酸钙溶于硝酸溶液CaCO3+2H+===Ca2++CO2↑+H2O(8)碳酸氢钙溶液与足量氢氧化钠溶液混合HCO-3+OH-===CO2-3+H2O第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/答案:序号正误判断错误原因正确的离子方程式(1)×产物应为Fe2+,不是Fe3+Fe+2H+===Fe2++H2↑(2)×产物为氢氧化铁胶体,不是沉淀Fe3++3H2O=====△Fe(OH)3(胶体)+3H+(3)×氢氧化铜不能写成离子的形式Cu(OH)2+2H+===Cu2++2H2O答案:序号正误判断错误原因正确的离子方程式(1)×产物应为Fe2+,不是Fe3+Fe+2H+===Fe2++H2↑(2)×产物为氢氧化铁胶体,不是沉淀Fe3++3H2O=====△Fe(OH)3(胶体)+3H+(3)×氢氧化铜不能写成离子的形式Cu(OH)2+2H+===Cu2++2H2O第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/序号正误判断错误原因正确的离子方程式(4)×应同时生成氢氧化铜和硫酸钡沉淀Ba2++2OH-+Cu2++SO2-4===BaSO4↓+Cu(OH)2↓(5)×离子方程式两边电荷不守恒2Al+3Cu2+===2Al3++3Cu序号正误判断错误原因正确的离子方程式(4)×应同时生成氢氧化铜和硫酸钡沉淀Ba2++2OH-+Cu2++SO2-4===BaSO4↓+Cu(OH)2↓(5)×离子方程式两边电荷不守恒2Al+3Cu2+===2Al3++3Cu第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/序号正误判断错误原因正确的离子方程式(6)×生成硫酸钡与水的物质的量之比应为1∶22H++SO2-4+Ba2++2OH-===BaSO4↓+2H2O(7)√--(8)×有碳酸钙沉淀生成Ca2++2HCO-3+2OH-===CaCO3↓+CO2-3+2H2O序号正误判断错误原因正确的离子方程式(6)×生成硫酸钡与水的物质的量之比应为1∶22H++SO2-4+Ba2++2OH-===BaSO4↓+2H2O(7)√--(8)×有碳酸钙沉淀生成Ca2++2HCO-3+2OH-===CaCO3↓+CO2-3+2H2O第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/4.下列各组离子能大量共存的是()A.无色透明溶液中:K+、Cu2+、Na+、SO2-4B.强碱性溶液中:K+、NH+4、Cl-、HCO-3C.能使酚酞溶液变红的溶液中:Na+、Cl-、CO2-3、NO-3D.含有Ba2+的酸性溶液中:Mg2+、Na+、SO2-4、S2-解析:选C。选项A中为无色溶液,Cu2+不可能大量存在;选项B中的OH-与HCO-3和NH+4不能大量共存;选项D中的Ba2+与SO2-4、H+与S2-不能大量共存。4.下列各组离子能大量共存的是()A.无色透明溶液中:K+、Cu2+、Na+、SO2-4B.强碱性溶液中:K+、NH+4、Cl-、HCO-3C.能使酚酞溶液变红的溶液中:Na+、Cl-、CO2-3、NO-3D.含有Ba2+的酸性溶液中:Mg2+、Na+、SO2-4、S2-解析:选C。选项A中为无色溶液,Cu2+不可能大量存在;选项B中的OH-与HCO-3和NH+4不能大量共存;选项D中的Ba2+与SO2-4、H+与S2-不能大量共存。第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/5.有一包白色粉末,其中可能含有硝酸钡、氯化钙、碳酸钾。现做以下实验:①将部分粉末加入水中,振荡,有白色沉淀生成;②向①的悬浊液中加入过量的稀硝酸,白色沉淀消失,并有气泡产生;③取少量②的溶液滴入硝酸银溶液中,有白色沉淀生成。根据上述实验现象,回答下列问题:5.有一包白色粉末,其中可能含有硝酸钡、氯化钙、碳酸钾。现做以下实验:①将部分粉末加入水中,振荡,有白色沉淀生成;②向①的悬浊液中加入过量的稀硝酸,白色沉淀消失,并有气泡产生;③取少量②的溶液滴入硝酸银溶液中,有白色沉淀生成。根据上述实验现象,回答下列问题:第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/(1)原白色粉末中肯定含有________(填化学式)。(2)写出硝酸钡与碳酸钾反应的离子方程式:____________________________________________________________________。(3)写出氯化钙与碳酸钾反应的离子方程式:____________________________________________________________________。(4)写出碳酸钙与硝酸反应的离子方程式:______________________________________________________________________。(1)原白色粉末中肯定含有________(填化学式)。(2)写出硝酸钡与碳酸钾反应的离子方程式:____________________________________________________________________。(3)写出氯化钙与碳酸钾反应的离子方程式:____________________________________________________________________。(4)写出碳酸钙与硝酸反应的离子方程式:______________________________________________________________________。第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/解析:①将部分粉末加入水中,振荡,有白色沉淀生成,白色沉淀可能是碳酸钡或碳酸钙,说明一定含有碳酸钾,其余两种中至少含有一种;②向①的悬浊液中加入过量稀硝酸,白色沉淀消失,并有气泡产生,证明沉淀是碳酸钙或碳酸钡;③取少量②的溶液滴入硝酸银溶液,有白色沉淀生成,说明含有氯离子,则混合物中一定含有氯化钙,故白色粉末中一定含有碳酸钾、氯化钙,可能含有硝酸钡。解析:①将部分粉末加入水中,振荡,有白色沉淀生成,白色沉淀可能是碳酸钡或碳酸钙,说明一定含有碳酸钾,其余两种中至少含有一种;②向①的悬浊液中加入过量稀硝酸,白色沉淀消失,并有气泡产生,证明沉淀是碳酸钙或碳酸钡;③取少量②的溶液滴入硝酸银溶液,有白色沉淀生成,说明含有氯离子,则混合物中一定含有氯化钙,故白色粉末中一定含有碳酸钾、氯化钙,可能含有硝酸钡。第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/答案:(1)K2CO3、CaCl2(2)Ba2++CO2-3===BaCO3↓(3)Ca2++CO2-3===CaCO3↓(4)CaCO3+2H+===Ca2++CO2↑+H2O答案:(1)K2CO3、CaCl2(2)Ba2++CO2-3===BaCO3↓(3)Ca2++CO2-3===CaCO3↓(4)CaCO3+2H+===Ca2++CO2↑+H2O第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/三、氧化还原反应方程式的配平1.步骤三、氧化还原反应方程式的配平1.步骤第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/2.方法(1)逆向配平法:如果氧化还原反应中氧化剂和还原剂是同一种物质(也称自身氧化还原反应),可以从生成物的一侧配平,即先确定生成物的系数,再确定反应物的系数。2.方法(1)逆向配平法:如果氧化还原反应中氧化剂和还原剂是同一种物质(也称自身氧化还原反应),可以从生成物的一侧配平,即先确定生成物的系数,再确定反应物的系数。第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/(2)残缺方程式的配平法:所谓残缺方程式,即某些反应物或生成物的化学式未写出,它们一般为化合价不变的水、酸、碱等。对于这类方程式的配平,不仅要配平化学计量数,还要写出未知物质的化学式,其方法是先配平含变价元素物质的化学计量数,再通过比较反应物和生成物,观察增、减的原子或离子数,从而确定未知物并配平。(3)合并配平法:某物质中元素的化合价同时升高或降低,可以将这种物质当作一个整体计算化合价变化总数。(2)残缺方程式的配平法:所谓残缺方程式,即某些反应物或生成物的化学式未写出,它们一般为化合价不变的水、酸、碱等。对于这类方程式的配平,不仅要配平化学计量数,还要写出未知物质的化学式,其方法是先配平含变价元素物质的化学计量数,再通过比较反应物和生成物,观察增、减的原子或离子数,从而确定未知物并配平。(3)合并配平法:某物质中元素的化合价同时升高或降低,可以将这种物质当作一个整体计算化合价变化总数。第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/【典例1】配平化学方程式H2S+HNO3―→S↓+NO↑+H2O。【典例1】配平化学方程式H2S+HNO3―→S↓+NO↑+H2O。第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/第三步:求总数,从而确定氧化剂(或还原产物)和还原剂(或氧化产物)的化学计量数。故H2S的化学计量数为3,HNO3的化学计量数为2。第三步:求总数,从而确定氧化剂(或还原产物)和还原剂(或氧化产物)的化学计量数。故H2S的化学计量数为3,HNO3的化学计量数为2。第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/第四步:配系数,先配平变价元素,再利用原子守恒配平其他元素。化学方程式为3H2S+2HNO3===3S↓+2NO↑+4H2O第五步:查守恒,利用O原子守恒来进行验证。第四步:配系数,先配平变价元素,再利用原子守恒配平其他元素。化学方程式为3H2S+2HNO3===3S↓+2NO↑+4H2O第五步:查守恒,利用O原子守恒来进行验证。第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/【典例2】Cu+HNO3(稀)―→Cu(NO3)2+NO↑+H2O。第一步:先配平含变价元素物质的化学计量数。该氧化还原反应中的变价元素为Cu和N,则有Cu(0)→Cu(+2)化合价升高2×3N(+5)→N(+2)化合价降低3×23Cu+HNO3(稀)―→3Cu(NO3)2+2NO↑+H2O。【典例2】Cu+HNO3(稀)―→Cu(NO3)2+NO↑+H2O。第一步:先配平含变价元素物质的化学计量数。该氧化还原反应中的变价元素为Cu和N,则有Cu(0)→Cu(+2)化合价升高2×3N(+5)→N(+2)化合价降低3×23Cu+HNO3(稀)―→3Cu(NO3)2+2NO↑+H2O。第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/第二步:根据原子守恒,用观察法确定其他物质的化学计量数。化学方程式为3Cu+8HNO3(稀)===3Cu(NO3)2+2NO↑+4H2O。第二步:根据原子守恒,用观察法确定其他物质的化学计量数。化学方程式为3Cu+8HNO3(稀)===3Cu(NO3)2+2NO↑+4H2O。第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/【针对训练】6.配平下列氧化还原反应方程式:(1)____HNO3=====光照____H2O+____NO2↑+____O2↑(2)______Cl2+______KOH===______KCl+______KClO3+______H2O答案:(1)4241(2)36513【针对训练】6.配平下列氧化还原反应方程式:(1)____HNO3=====光照____H2O+____NO2↑+____O2↑(2)______Cl2+______KOH===______KCl+______KClO3+______H2O答案:(1)4241(2)36513第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/7.配平下列方程式:(1)________MnO-4+________Fe2++________H+===________Mn2++________Fe3++________H2O(2)________P+________FeO+________CaO=====高温________Ca3(PO4)2+________Fe(3)________KI+________KIO3+________H2SO4===________I2+________K2SO4+________H2O(4)________MnO-4+________H++________Cl-===________Mn2++________Cl2↑+________H2O7.配平下列方程式:(1)________MnO-4+________Fe2++________H+===________Mn2++________Fe3++________H2O(2)________P+________FeO+________CaO=====高温________Ca3(PO4)2+________Fe(3)________KI+________KIO3+________H2SO4===________I2+________K2SO4+________H2O(4)________MnO-4+________H++________Cl-===________Mn2++________Cl2↑+________H2O第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/答案:(1)158154(2)25315(3)513333(4)21610258答案:(1)158154(2)25315(3)513333(4)21610258第一章 物质及其变化PPT模板:www.1ppt.com/moban/PPT素材:www.1ppt.com/sucai/PPT背景:www.1ppt.com/beijing/PPT图表:www.1ppt.com/tubiao/PPT下载:www.1ppt.com/xiazai/PPT教程:www.1ppt.com/powerpoint/资料下载:www.1ppt.com/ziliao/范文下载:www.1ppt.com/fanwen/试卷下载:www.1ppt.com/shiti/教案下载:www.1ppt.com/jiaoan/PPT论坛:www.1ppt.cnPPT课件:www.1ppt.com/kejian/语文课件:www.1ppt.com/kejian/yuwen/数学课件:www.1ppt.com/kejian/shuxue/英语课件:www.1ppt.com/kejian/yingyu/美术课件:www.1ppt.com/kejian/meishu/科学课件:www.1ppt.com/kejian/kexue/物理课件:www.1ppt.com/kejian/wuli/化学课件:www.1ppt.com/kejian/huaxue/生物课件:www.1ppt.com/kejian/shengwu/地理课件:www.1ppt.com/kejian/dili/历史课件:www.1ppt.com/kejian/lishi/本部分内容讲解结束



















